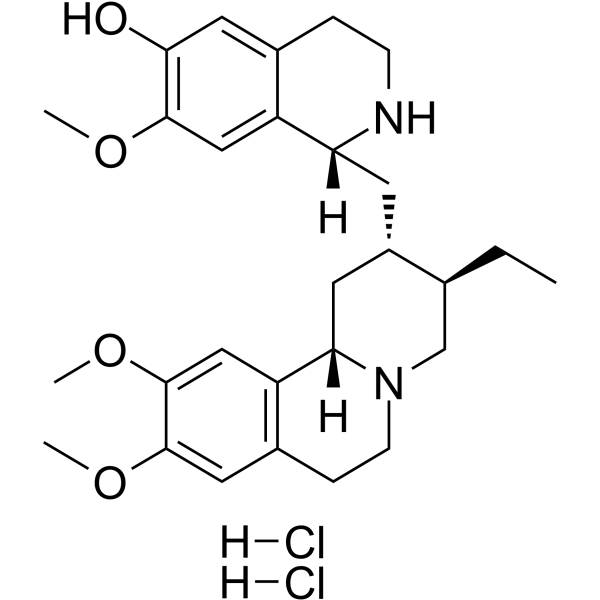
Cephaeline Dihydrochloride
CAS No. 5853-29-2
Cephaeline Dihydrochloride ( —— )
产品货号. M31773 CAS No. 5853-29-2
(-)-Cephaeline (dihydrochloride) is an enantiomer of Cephaeline. Cephaeline is a selective CYP2D6 inhibitor (IC50: 121 μM).
纯度: >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| 规格 | 价格/人民币 | 库存 | 数量 |
| 50MG | 获取报价 | 有现货 |


|
| 100MG | 获取报价 | 有现货 |


|
生物学信息
-
产品名称Cephaeline Dihydrochloride
-
注意事项本公司产品仅用于科研实验,不得用于人体或动物的临床与诊断
-
产品简述(-)-Cephaeline (dihydrochloride) is an enantiomer of Cephaeline. Cephaeline is a selective CYP2D6 inhibitor (IC50: 121 μM).
-
产品描述(-)-Cephaeline (dihydrochloride) is an enantiomer of Cephaeline. Cephaeline is a selective CYP2D6 inhibitor (IC50: 121 μM).
-
体外实验CYP2D6 reveals the highest metabolic activity for the generation of 9-O-demethylEmetine, whereas this enzyme also shows a significant metabolic activity for the generation of Cephaeline. The IC50s of Cephaeline against CYP2C9, CYP2D6 and CYP3A4 is over 1000, 121 and 1000 μM, respectively. Further experiments are performed to determine inhibition constants (Ki) for Cephaeline on the CYP2D6 and CYP3A4 activities Graphic analysis of Dixon plots at various Cephaeline concentrations for each of the two CYP enzyme assays yield Kis of 54 and 355 μM for CYP2D6 and CYP3A4, respectively.
-
体内实验——
-
同义词——
-
通路Others
-
靶点Other Targets
-
受体——
-
研究领域——
-
适应症——
化学信息
-
CAS Number5853-29-2
-
分子量539.53
-
分子式C28H40Cl2N2O4
-
纯度>98% (HPLC)
-
溶解度In Vitro:?DMSO : 250 mg/mL (463.37 mM)
-
SMILES——
-
化学全称——
运输与储存
-
储存条件(-20℃)
-
运输条件With Ice Pack
-
稳定性≥ 2 years
参考文献
产品手册




关联产品
-
SAH
SAH 是 METTL3-METTL14 异二聚体复合物的抑制剂(METTL3-14,IC50:0.9 μM)。
-
Hydrastine
Hydrastine 是一种天然生物碱,存在于Hydrastis Canadensis 中。
-
2-Methyl-1-Pyrroline
2-Methyl-1-Pyrroline (2-MPN) is an intermediate of a photochemical dye that induces the formation of (R)-2-MP.



 021-51111890
021-51111890 购物车()
购物车()
 sales@molnova.cn
sales@molnova.cn







